Magnetic Resonance in the Solid State: Magic-angle Spinning NMR and Dynamic Nuclear Spin Polarization
Associated Research Group Prof. Björn Corzilius (Universität Rostock)
Nuclear magnetic resonance (NMR) allows to resolve the short-range order between neighboring atomic nuclei, and thus to elucidate the structure of molecules and solids at the atomic level. Here, in comparison to other methods such as X-ray diffraction, no long-range order is necessary. Thus, solid-state NMR is the methodology of choice for the investigation of low-order samples such as (bio)polymers, colloidal suspensions, or amorphous solids. In combination with dynamic nuclear spin polarization (DNP), the surfaces of solids and materials can be analyzed particularly selectively.
Compared to NMR in solution, internal or anisotropic interactions of nuclear spins typically dominate in solids, i.e. interactions that depend on the orientation of the molecular or crystalline system to the external magnetic field. This represents an enormous potential of information, but at the same time provides a large linewidth of the nuclear magnetic resonance signals in the NMR spectrum. For this reason, we use the method of magic-angle spinning, in which the sample is rotated at a "magic angle" of 54.74° to the external magnetic field at rotational frequencies up to 67 kHz. This averages out these interactions almost completely and improves the resolution enormously; at the same time, however, they can be reintroduced in a controlled manner by radio frequency pulse sequences in order to obtain the valuable information about the spin system and thus the atomic structure.
Under typical NMR conditions, the occupation difference of the nuclear spin states is very small: only about 1 in 10,000 spins effectively contributes to the measurable NMR signal. This is due to the nearly equal occupancy of their ground state (α) and excited state (β). Electron spins have a much larger magnetic moment and thus a larger splitting energy under the same conditions (magnetic field and temperature), so that an occupation difference of about 6% occurs here. By using special instrumentation, this occupation difference can be transferred to the nuclear spins by irradiating high-frequency microwaves, thus producing ~100-1000-fold signal enhancement in the NMR spectrum. For this dynamic nuclear polarization (DNP) we use a 263 GHz gyrotron as microwave source and can lower the sample temperature to about 100 K by a special cryo-MAS system.[1]
In a collaboration with the Brückner group we investigate the structure-reactivity relationships of supported Ni/SiO2-Al2O3-catalysts in olefin oligomerization. Here, we concertedly apply different magnetic resonance methods such as solid-state NMR, (operando) EPR, as well as DNP-enhanced MAS NMR.
In many years of research on butene dimerization with Ni/SiO2-Al2O3 catalysts [2] [3], it was found that activity and selectivity crucially depend on two properties, 1) the Brønsted acidic centers (BA) of the supports and 2) the structure of the Ni species. BAs are on the one hand required to stabilize individual Ni centers on the carrier and prevent the formation of inactive Ni clusters, but on the other hand catalyze the undesired isomerization to highly branched and longer chain products >C8. In the case of Ni/SiO2-Al2O3 catalysts, the process shown in the following figure was proposed upon impregnation with Ni(COD)2 or Ni(cp)2 and supported by operando-EPR or FTIR of adsorbed CO.[2]
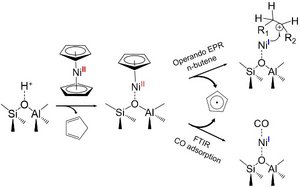
However, it remains unclear which carrier atoms are preferentially involved in the immediate coordination of the Ni centers (Si or Al) and how this affects the electronic and structural properties as well as the catalytic behavior.
The interaction of the various magnetic resonance methods will create a broad spectroscopic portfolio that can be used to answer specific mechanistic questions about the activity and selectivity of heterogeneous catalysts.
Literatur
[1] Lilly Thankamony, A. S.; Wittmann, J. J.; Kaushik, M.; Corzilius, B. Dynamic Nuclear Polarization for Sensitivity Enhancement in Modern Solid-State NMR. Prog. Nucl. Magn. Reson. Spectrosc. 2017, 102-103, 120-195.
[2] Rabeah, J.; Radnik, J.; Briois, V.; Maschmeyer, D.; Stochniol, G.; Peitz, S.; Reeker, H.; La Fontaine, C.; Brückner, A. Tracing Active Sites in Supported Ni Catalysts during Butene Oligomerization by Operando Spectroscopy under Pressure. ACS Catalysis 2016, 6, 8224-8228.
[3] Vuong, T. H.; Rockstroh, N.; Bentrup, U.; Rabeah, J.; Knossalla, J.; Peitz, S.; Franke, R.; Brückner, A. Role of Surface Acidity in Formation and Performance of Active Ni Single Sites in Supported Catalysts for Butene Dimerization: A View inside by Operando EPR and In Situ FTIR Spectroscopy. ACS Catalysis 2021, 11, 3541-3552.
