Telomerization reactions
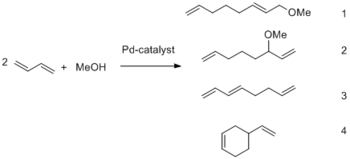
During the course of our studies into palladium-catalyzed C–C coupling reactions we became interested in the telomerization of 1,3-butadiene. This reaction assembles simple starting materials in a 100 % atom efficient manner to give octadienes. In general, the telomerization reaction is the dimerization of two molecules of a 1,3-diene in the presence of an appropriate nucleophile HX, e.g. alcohols, to give substituted octadienes (1-substituted 2,7-octadiene, 3-substituted 1,7-octadiene). The resulting compounds are useful as intermediates in the total synthesis of several natural products as well as in industry, as precursors for plasticizer alcohols, important monomers, solvents, corrosion inhibitors and non-volatile herbicides. From an industrial point of view 1,3-butadiene and methanol (Scheme 1) are the most attractive starting materials for this reaction due to their ready availability and their exceedingly low price. Therefore our main interests are investigations in this direction.
Literature
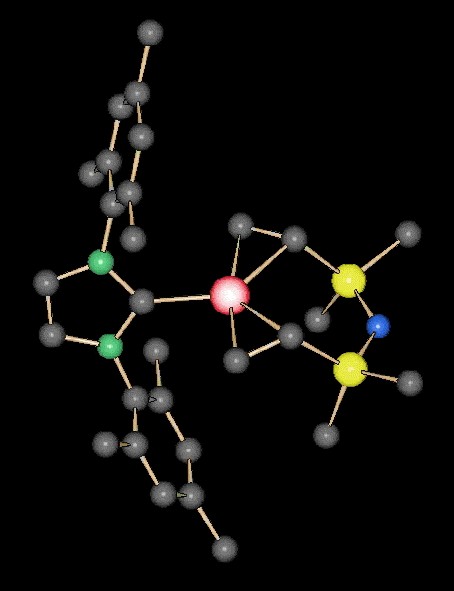
R. Jackstell, M. G. Andreu, A. Frisch, K. Selvakumar, A. Zapf, H. Klein, A. Spannenberg, D. Roettger, O. Briel, R. Karch, M. Beller, Angew. Chem. Int. Ed. 2002, 41, 986-989, A Highly Efficient Catalyst for the Telomerization of 1,3-Dienes with Alcohols: First Synthesis of a Monocarbenepalladium(0)-Olefin Complex
R. Jackstell, S. Harkal,H. Jiao, A. Spannenberg, C. Borgmann, D. Röttger,F. Nierlich, M. Elliot, S. Niven, K. Cavell,O. Navarro, M. S. Viciu, S. P. Nolan, M. Beller; Chem. Eur. J. 2004, 10, 3891-3900 An Industrially Viable Catalyst System for Palladium-Catalyzed Telomerizations of 1,3-Butadiene with Alcohols