Hydroformylation reactions
The selective hydroformylation of aliphatic Olefins to Linear Aldehydes is one of the most homogeneous catalytic reactions in the industry. More than 8 million tons of various aldehydes and alcohols are produced annually in this way. The hydroformylation of propene is e.g. important for the production of n-butanal which serves as starting material for 2-ethylhexanol, the most important plasticizer alcohol. Lately the interest in alternative plasticizer alcohols to the status quo of ecological interest (biodegradability, other feedstocks ...) is growing. For economic (and environmental) reasons make olefin mixtures terminal and internal olefins very suitable starting materials for new plasticizer alcohols, which fall should be linear as possible because of their physical properties. Selective hydroformylation of internal olefins to linear aldehydes must be done with suitable catalysts wich can isomerize both, as well as highly selective hydroformylate only terminal. So we are developing new rhodium- and rutheniumbased catalysts which are capable of highly selective hydroformylate both terminal and internal Olefins.
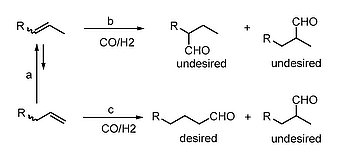
Besides the fact that rhodium is considered "state of the art" metal for hydroformylations, there is a considerable need for research into less expensive hydroformylation. This object today represents an important current topic in catalysis research and provides interesting industrial applications. Our interest lies in the development of new catalyst systems using less common metals such as iridium, palladium and ruthenium, which had been underestimated until now in this reaction and are.
Literature
R. Jennerjahn, I. Piras, R. Jackstell, R. Franke, K. D. Wiese, M. Beller, Chem. Eur. J. 2009, 15, 6383-6388, Palladiumcatalysed Isomerization and Hydroformylation of Olefins
I. Piras, R. Jennerjahn, R. Jackstell, A. Spannenberg, R. Franke, M. Beller, Angew. Chem. int. Ed, 2011, VIP, insidecover, 50, 280-284A General and Efficient Iridium-catalyzed Hydroformylation of Olefins
I. Fleischer, K. Dyballa, R. Jennerjahn, R. Jackstell, R. Franke, A. Spannenberg, M. Beller, Angew. Chem. Int. Ed, 2013, 52(10), 2949-2953, “From olefins to alcohols: Efficient and regioselective Rutheniumcatalysed Domino Hydroformylation/reduction sequence”
X. Fang, M. Zhang, R. Jackstell, M. Beller, Angew. Chem. Int. Ed. 2013, 52, 17, 4645-4649, Selective Palladium-Catalyzed Hydroformylation of Alkynes to α,β-Unsaturated Aldehydes
L. Wu, I. Fleischer, R. Jackstell, I. Profir, R. Franke, M. ; Beller, Matthias, J. Am. Chem. Soc. 2013, 135, 14306-14312 :Ruthenium-Catalyzed Hydroformylation/ Reduction of Olefins to Alcohols: Extending the Scope to Internal Alkenes

