Catalysis for energy
Dr. Henrik Junge
Forced by political decisions, intensive work is currently being done in Germany on the development of hydrogen technologies. Already since 2003, the group "Catalysis for Energy Technologies" has been working on the production of (green) hydrogen from renewable energies and raw materials, the chemical storage of hydrogen and its use for carbon dioxide utilization through the synthesis of energy carriers and industrially relevant products (e.g. carbon monoxide, formic acid, methanol, methyl formate). For this purpose, classical hydrogenation/dehydrogenation reactions as well as photocatalytic reactions are applied. To improve these conversions, we combine our methodological expertise in reaction design and product analysis with our experience in the development of active and selective catalysts and photosensitizers. Both, organometallic complexes as well as heterogeneous catalysts, such as supported nanoparticles and "single atoms", are used as catalysts. In principle, besides noble metals also non-precious metals play an important role. In all research topics and projects we cooperate/were cooperating internationally with leading groups and industrial companies. The individual topics specifically include:
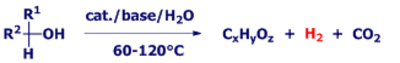
The aim of our work is the development of appropriate and active catalysts that allow for the hydrogen generation from starting materials like methanol, bioethanol, glycerol, glucose and cellulose with high selectivities at ambient conditions. Here, especially the generation of carbon monoxide shall be avoided since this constitutes a poison for fuel cell catalysts. In addition to hydrogen, other valuable products like acetic acid, its ethyl ester, propandiol acetone and lactic acid are accessible. A recent highlight was the successful application of one of our catalyst systems in continuous low-temperature hydrogen production from methanol/water over 450 h in a demonstration plant at FAU Erlangen-Nuremberg (Metha-Cycle project, funded by the BMWi).
Ref.:
- M. Nielsen, E. Alberico, W. Baumann, H.-J. Drexler, H. Junge, S. Gladiali, M. Beller, Nature 2013; 495, 85-90; Low-temperature aqueous-phase methanol dehydrogenation to hydrogen and carbon dioxide.
- P. Zhang, Y.-J. Guo, J. Chen, Y.-R. Zhao, J. Chang, H. Junge, M. Beller, Y. Li, Nature Catalysis 2018, 1, 332.
- C. H. Schwarz, D. Kraus, E. Alberico, H. Junge, M. Haumann, EJIC. 2021, 1745-1751, Immobilized Ru-Pincer Complexes for Continuous Gas Phase Low-Temperature Methanol Reforming - Improving the Activity by a Second Ru-Complex and Variation of Hydroxide Additives.
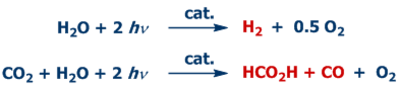
The direct photocatalytic generation of hydrogen, carbon monoxide, formic acid and other products using (sun)light is the focus of our work.
The conversion of the (bio)formic acid obtained by photocatalysis or produced from biomass to methanol, methyl formate and oxymethylene ethers is another focus of our investigations. Also in these areas we are working on the development of sufficiently available catalyst materials that are free of precious metals.


Ref.:
- C. Steinlechner, A. F. Rösel, E. Oberem, A. Päpcke, N. Rockstroh, F. Gloaguen, S. Lochbrunner, R. Ludwig, A. Spannenberg, H. Junge, R. Francke, M. Beller, ACS Catal. 2019, 9, 2091-2100; Selective Earth-Abundant System for CO2 Reduction: Comparing Photo- and Electrocatalytic Processes.
- J. Schneidewind, Miguel A. Argüello Cordero, H. Junge, S. Lochbrunner, M. Beller, Energy Envir. Sci. 2021, 14, 4427-4436; Two-photon, visible light water splitting at a molecular ruthenium complex.
- E. Alberico, T. Leischner, H. Junge, A. Kammer, R. Sang, J. Seifert, W. Baumann, A. Spannenberg, K. Junge, M. Beller, Chem. Sci. 2021, 12, 13101-13119; HCOOH Disproportionation to MeOH promoted by Molybdenum PNP Complexes.
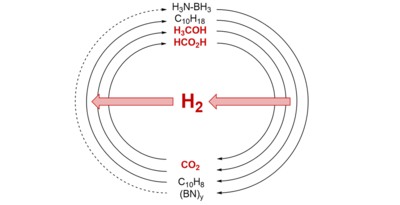
The highly efficient and long-term storage of hydrogen is a key issue in hydrogen technology. Based on the combination of catalytic production and decomposition of liquid energy and hydrogen carriers, cycles for CO2-neutral hydrogen storage are possible. Among others such cycles are conceivable for formic acid, its salts, the formates, methanol and methyl formate. In all cases, the high volumetric energy density of the liquids or solutions compared to that of hydrogen is exploited.
A special role is also played by the availability and concentration of carbon dioxide, which should at best be separated directly from air by means of suitable absorbents and converted into downstream products. In this context, e.g. amino acid-based systems are efficient materials for CO2 capture and nevertheless allow the subsequent hydrogenation of the captured carbon dioxide to alternative energy carriers such as formates.
In addition to the development of new innovative catalysts based on readily available metals, a major goal is to realize demonstrators for pilot plant scale.

Ref.:
- A. Boddien, D. Mellmann, F. Gärtner, R.Jackstell, H. Junge, P. J. Dyson, G. Laurenczy, R.Ludwig, M. Beller, Science, 2011, 333, 1733-1736; Efficient Dehydrogenation of Formic Acid using an Iron Catalyst.
- D. Wei, H. Junge, M. Beller, Chem. Sci. 2021, 12, 6020-6024; An amino acid based system for CO2 capture and catalytic utilization to produce formates.
- D. Wei, R. Sang, P. Sponholz, H. Junge, M. Beller, Nature Energy, 2022, DOI: 10.1038/s41560-022-01019-4; A carbon-neutral chemical hydrogen battery based on formic acid.
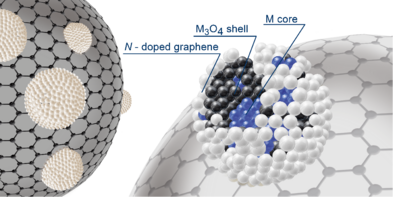
In this subfield again the focus is on the development of catalyst materials that are free of precious metals and sufficiently available to replace expensive precious metals. Here, the stabilization of metal clusters and nanoparticles is achieved by binding to donor atoms incorporated in suitable supports. Applications include the selective dehydrogenation of formic acid using cobalt catalysts and the electrochemical reduction of carbon dioxide to carbon monoxide using an iron catalyst. Structural analysis has demonstrated the formation of finely distributed MNx species for each of these compounds, as well as their activity in the target reaction.
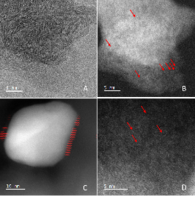
Ref.:
- C. Tan, A.-E. Surkus, F. Chen, M.-M. Pohl, G. Agostini, M. Schneider, H. Junge, M. Beller, Angew. Chem. 2017, 129, 16843-16847, Angew. Chem. Int. Ed. 2017, 56, 16616-16620; A Stable Nano-Cobalt Catalyst for Selective Dehydrogenation of Formic Acid with Highly Dispersed CoNx Active Sites.
- X. Li, A.-E. Surkus, J. Rabeah, M. Anwar, S. Dastigir, H. Junge, A. Brückner, M. Beller, Angew. Chem. 2020, 132, 15983-15988, Angew. Chem. Int. Ed. 2020, 59, 15849-15854; Cobalt Single-Atom Catalysts with High Stability for Selective Dehydrogenation of Formic Acid.
- M. Miola, S. Li, X.-M. Hu, M. Ceccato, A.-E. Surkus, E. Welter, S. U. Pedersen, H. Junge, T. Skrydstrup, M. Beller, K. Daasbjerg, Advanced Materials Interfaces, 2021, 2100067; Highly scalable conversion of blood protoporphyrin to efficient electrocatalyst for CO2-to-CO conversion.



