Polymer Chemistry & Catalysis
Prof. Esteban Mejìa
C-H Bond Generation and Derivatization through Nonconventional, Green Catalytic Methods
The development of carbon-based and noble metal-free catalysts has been the focus of many research efforts during the last decades due to the environmental and toxicologic concerns associated with the widespread use of noble metal-based catalysts and the increasing urge towards sustainable chemical processes. In recent years, guided by Green Chemistry’s principles, the application of catalytic technologies towards the development of sustainable processes and products has been the main research motivation at the Mejía’s group. Our main research activities focus on: C-C bond formation via C-H derivatization under oxidative conditions (ultimately using oxygen as oxidant) and C-H bond generation using heterogeneous catalysts derived from biowaste. These goals have been achieved by the exploitation of the redox reactivity of molecular catalysts, as well as their incorporation in novel porous materials (synthetic and bio-derived).
These research activities have been possible thanks to the funds acquired from different agencies including: the German Federal Ministry of Education and Research (BMBF), the German Academic Exchange Service (DAAD), the German Federal Ministry for Economic Cooperation and Development (BMZ), and the Leibniz Society. Moreover, thanks to very fruitful industrial cooperation, 25 patents have been filed worldwide. Some of the catalysts and processes protected therein have been transferred to an industrial multi-ton scale
C-C bond forming reactions are at the hearth of many important transformations both in industry and academia. From the myriad of catalytic approaches to achieve these transformations, those relying on C-H functionalization are gaining increasing interest due to their inherent sustainable nature. These processes are atom-economic, make synthesis protocols shorter and reduce the amount of produced waste (metal salts), leading to sustainable processes, an ultimate goal of our modern society in which environmental concerns are of highest priority.
Very recently, we reported the copper-catalyzed alkynylation of underivatized allylic substrates under oxidative conditions using terminal alkynes (Fig. 1).1 This is an example of a thermal cross-dehydrogenative coupling (CDC) reaction, which can be described as the coupling of a pre-nucleophile and a pre-electrophile, both devoid of leaving groups. Later on, we combined for the first time the advantages of CDC and photocatalysis for the alkynylation of allylic substrates, using a copper photocatalyst under much milder conditions that before.2
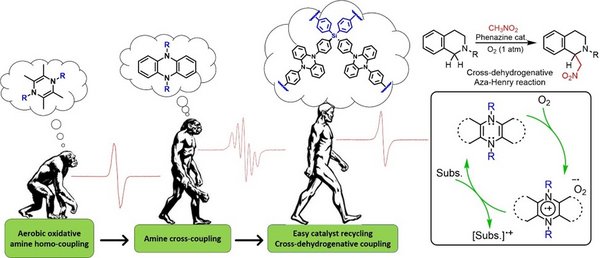
Recently, our group reported a novel synthetic methodology for the synthesis of the elusive 1,4-functional tetramethylpyrazines (Fig. 2, “ape”) and used them as catalysts for the aerobic oxidative homocoupling of primary amines, thanks to the reversible redox behavior of these compounds and the remarkable stability of their radical-cationic form.3 We explored further the exploitation of stable radical cations as oxidation catalysts with the development of a series of 5,10-disubstitued phenazines (Fig. 2, “homo habilis”) which showed remarkable activities for the oxidative homo and cross coupling of amines.4 Interestingly, even though the strong redox properties of phenazines and the stability of their open-shell derivatives are relatively well-known, there are only few additional reports on their use as redox catalysts. Inspired by our previous works on the preparation of hybrid porous polymers based on polyhedral oligomeric silsesquioxane (POSS),5 and the aforementioned pyrazine and phenazine radical-cation oxidation catalysts,4 we designed and synthesized a novel type of covalently linked porous polymers exhibiting tetrakis(phenyl)silane moieties as structural elements cross-linked by phenazine groups (Fig. 2, “homo sapiens”).
Moreover, we extended the reaction scope of this type of catalysts and used successfully for C-C bond formations via cross-dehydrogenative Aza-Henry reactions. This represents, to the best of our knowledge, the first example of a metal-free heterogeneous system for cross-dehydrogenative coupling reactions.6
[1] A. A. Almasalma and E. Mejia, Chem. Eur. J., 2018, 24, 12269-12273. [2] A. A. Almasalma and E. Mejia, Synthesis, 2020, 52, 529-536. [3] R. Brisar, D. Hollmann and E. Mejia, Eur. J. Org. Chem., 2017, 2017, 5391-5398. [4] R. Brišar, F. Unglaube, D. Hollmann, H. Jiao and E. Mejía, J. Org. Chem., 2018, 83, 13481-13490. [5] D. Wang and E. Mejia, ChemistrySelect, 2017, 2, 3381-3387. [6] F. Unglaube, P. Huenemoerder, X. Guo, Z. Chen, D. Wang and E. Mejia, Helv. Chim. Acta, 2020, 103, e2000184.
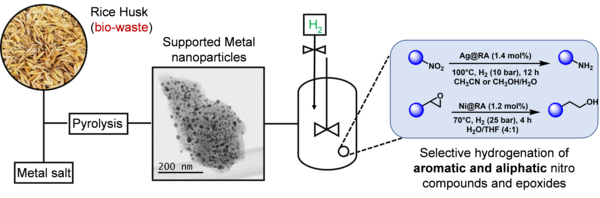
The development of strategies for the sustainable management and valorization of agricultural waste is of outmost importance. With this in mind, we have assessed the use of rice husk (RH) as feedstock for the preparation of heterogeneous catalysts containing metal nanoparticles of silver, cobalt, and nickel (Fig. 3).7 The inherent complexity of waste biomaterials has been exploited, conferring the prepared catalysts particular properties which are otherwise unachievable by synthetic means, including composition, morphology, stability, etc. These novel bio-genic metal-based catalysts showed excellent activity and remarkable selectivity in the hydrogenation of nitro and epoxide groups in both aromatic and aliphatic substrates, even in the presence of reactive functionalities like halogens, carbonyls, borate esters or nitriles. Recycling experiments showed that the catalysts can be easily recovered and reused multiple times without significant drop in performance and without requiring re-activation. Importantly, this alternative biomass valorization approach.
We foresee that the implementation of this methodology within a bio-refinery concept in rice-farming communities is feasible within a reachable timescale (which is the overall goal of the consortium this project belongs to). The success of said bio-refinery can effectively tackle some of the most relevant sustainable development goals (SDG) for agricultural societies, addressing economic growth, innovation, industrialization, and improvement of production and recycling patterns, while reducing the nefarious footprint of human activities on the planet.
[7] (a) F. Unglaube, J. Schlapp, A. Quade, J. Schäfer and E. Mejía, Catalysis Science & Technology, 2022, DOI: 10.1039/D2CY00005A; (b) F. Unglaube, C. R. Kreyenschulte and E. Mejia, ChemCatChem, 2021, 13, 2583-2591