Bio-based carbonates and hydroxycarbamates
The chemical conversion of CO2 gained more and more important in recent years. Thus, the effect of CO2 as a greenhouse gas can be reduced only marginally since its industrial use of 110 million metric tons per year comprises less than 1% of the annual emissions. But CO2 is a cheap, non-toxic and readily available carbon source. Its lack of chemical reactivity is the main obstacle so far to a broad range of chemical applications. However, the reaction of epoxides and CO2 which is thermodynamically allowed leads to sufficient conversions and yields using suitable catalysts [1].
In recent years, we have been investigated the preparation of fatty acid-based carbonates in cooperation with the thematic group "Gas phase oxidations" of our research department (Fig. 1). These carbonates (triglycerides or alkyl esters) are novel products which might be used as plasticizers, lubricants or precursors for polymers. They were produced in autoclaves under high pressure (100 bar) at 100-115 °C. A screening of various catalysts as well as the optimization of the reaction conditions was carried out.
Alkali metal halides, in particular KI, were found to be the most active and selective catalysts in combination with polyglycols or crown ethers as phase transfer catalysts (PTC) [2].
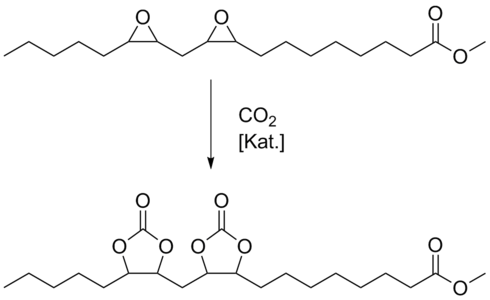
Potassium iodide together with polyglycol PEG 400 as PTC converted the epoxidized soybean oil to the respective carbonated triglyceride up to 84% and a selectivity of 99% referring to the carbonate formed. The results showed that both the nature of the halide anion and the alkali metal cation affected the carbonation. The solubility of the catalyst represented the major influencing factor. While KI and various polyglycols of different chain length formed very active catalyst systems, NaI was completely inactive. Due to the poor solubility of KBr, a larger amount of a PTC had to be used in order to enable a satisfying reaction.
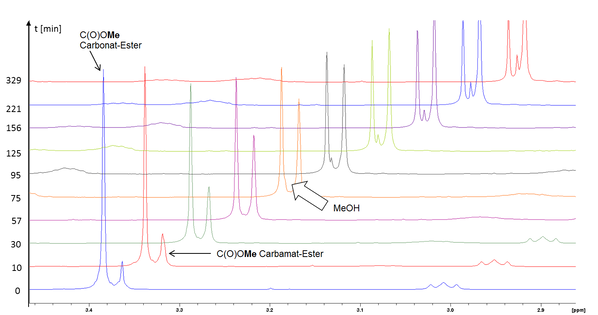
Carbonatized methyl oleate reacted with various primary and secondary amines to hydroxy-carbamates ([3], Fig. 2) for the first time. Especially, primary aliphatic amines were as reactive that conversions to methyl 9(10)-aminocarbonyloxy-10(9)-hydroxy-stearates could be performed at room temperature. However, a drawback of this high reactivity was an undesirable side reaction, the aminolysis of the methyl ester group to the corresponding fatty amide, which increased with rising chain length of the amine. Already after 75 min, the formation of the amide could be detected in an in situ NMR experiment by appearance of the signal for released methanol at about 3.36 ppm during the reaction of carbonated methyl oleate and n-decyl amine (Fig. 3, ratio carbonate/amine 1:1).
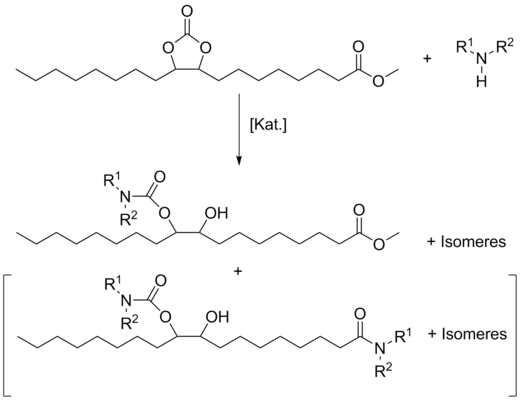
This amide formation occurred in the reaction with secondary amines only in case of piperidine. Secondary amines required more drastic reaction conditions in autoclaves.
Some of the new fatty ester-based hydroxy-carbamates were tested as anti-wear additives or friction modifiers in lubricating oils.
The studies on the carbonation of fatty acids were carried out in cooperation between the thematic groups "Gas phase oxidations" and "Liquid phase oxidations" (Dr. M. Polyakov, Dr. A. Köckritz) of our research department and the research department of Prof. Brückner in cooperation with Evonik Industries and the working groups of Prof. D. Agar (Dortmund), Prof. A. Pfennig (Graz) and Prof. W. Arlt (Erlangen, Germany). The aminolysis of the carbonates was performed in cooperation with Evonik Industries.
Reference
[1] T. Sakakura, K. Kohno, Chem. Commun. 1312 (2009).
Publications
[2] B. Schäffner, M. Blug, D. Kruse, M. Polyakov, A. Köckritz, A. Martin, P. Rajagopalan, U. Bentrup, A. Brückner, S. Jung, D. Agar, B. Rüngeler, A. Pfennig, K. Müller, W. Arlt, B. Woldt, M. Graß, S. Buchholz, ChemSusChem 7, 1133-1139 (2014).
[3] A. Symank, Master Thesis, Rostock University, 2014.
