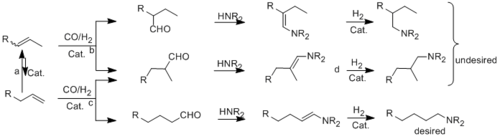
Metal catalyzed hydroaminomethylation reactions
An environmentally benign (atom-efficient, one-pot) synthesis of amines from olefins is the so-called hydroaminomethylation reaction. This domino reaction consists of initial hydroformylation of the olefin to aldehyde and subsequent formation of enamine (or imine) followed by hydrogenation. In order to obtain linear amines from internal olefins via hydroaminomethylation, a suitable catalyst has to fulfill a number of requirements. It must catalyze fast isomerization between the internal and terminal olefins. Since the thermodynamic equilibrium mixture generally contains less than 5% of the terminal olefin, the hydroformylation of the terminal olefin must occur faster and with high n-selectivity compared to the reaction of the internal olefin. Moreover, the catalyst must be active and selective for enamine (or imine) hydrogenation. Our interests are the realization and optimization of the selective hydroaminomethylation of internal and terminal (also functionalyzed and longerchained, aliphatic and aromatic) olefins by using derivatives of different chelating phosphorous ligands. Our current focus is on the use of cheap metal ruthenium in these reactions.
Literature
A.Seayad, M. Ahmed, H. Klein, R. Jackstell, T. Gross, M. Beller, SCIENCE 2002, 297 (5587), 1676-1678
L. Wu, I. Fleischer, R. Jackstell, M. Beller, J. Am. Chem. Soc. 2013, 135,10, 3989-3996, „Efficient and regioselective Ruthenium-catalysed Hydroaminomethylation of Olefins”
S. Gülak, L. Wu, Q. Liu, R. Franke, R. Jackstell, M. Beller, Angew. Chem. Int. Ed. 2014, 53, 7320-7323, Phosphine- and Hydrogen-Free Highly Regioselective Ruthenium-catalyzed Hydroaminomethylation of Olefins